Over the next several weeks — leading up to our planned launch — we’ll be sharing transparent behind-the-scenes updates on how we’re building, testing, and preparing the PetsPEMF RollnRest device for pets.
This journal will cover every major part of the development process:
• textile and foam engineering
• coil design and PEMF field validation
• electronics integration
• durability testing for daily pet use
• certification steps
• app updates
• and the final preparations for production
We’ll be updating this page frequently so you can follow the journey in real time.
Marko Kadunc, CEO
For any questions, you are welcome to contact me directly at marko.kadunc@omnipemf.com
Development Timeline
(Newest Updates First)
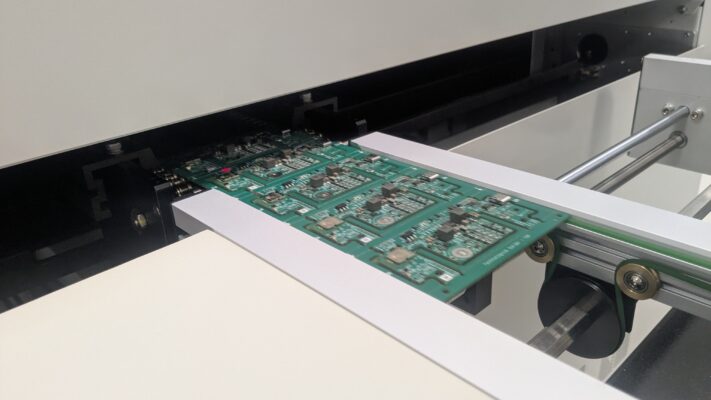
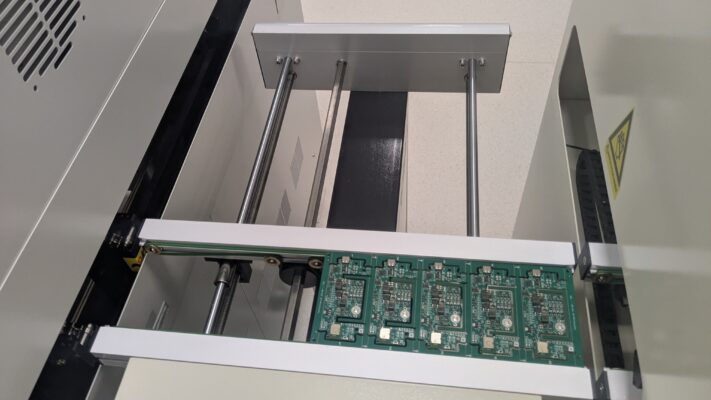
Date: February 23rd, 2026: RollnRest Production Update – Assembly Underway
We’re thrilled to share that we’ve reached another major milestone.
✔ Assembly in Progress
After months of development, refinement, and careful preparation, the technology is coming together in its final form – designed to bring calm, comfort, and balance to your pets’ daily lives.
Seeing the first units take shape is an incredibly exciting moment for our entire team.
✔ Packing
At the same time, we are preparing the packaging for shipment. Each RollnRest unit is being carefully packed and quality-checked to ensure it arrives perfectly protected and ready to use the moment it reaches your home.
We’ve attached a few photos from the production floor so you can follow the progress with us.
✔ Next Step: Shipping
As previously announced, the next step is shipping and delivery. We are now entering the final stage before RollnRest begins its journey to you and to the pets who will soon be enjoying its soothing effect. To every pet owner who supported us, followed the development, and believed in this product – thank you. Your trust and patience mean everything to us.
We can’t wait for RollnRest to be in your space… and even more importantly, in your pet’s favorite resting spot.

Date: February 16th, 2026: RollnRest Production Update – Preparing for Shipment
We’re excited to share that everything is moving according to plan and we’re getting very close to shipping.
✔ Full Production in Progress
PCB production and components are in place, and we are now getting ready for assembly. Watching everything come together for your pets’ comfort is a huge milestone for us.
✔ Next Step: Assembly & Packing
Once assembly ramps up, the next stage will be packing – preparing each RollnRest unit for delivery. Every step is handled with care to ensure it arrives ready to help your pets relax, recover, and feel safe. We’ve attached a few photos directly from the factory so you can take a look behind the scenes. It’s incredibly exciting for us to see everything coming together.
Thank you again for your patience, excitement, and trust throughout this journey. The wait is almost over.
Get ready! RollnRest is almost on its way.
Date: February 9rd, 2026: RollnRest Shipping Update – It’s Finally Happening!
The moment you’ve all been waiting for is almost here… and we couldn’t be more thrilled to share it with you!
✔ Shipping Launch: February 25th
After months of meticulous production and preparation, we are officially ready to start shipping RollnRest! The first wave of deliveries kicks off on February 25th.
✔ High Demand – Priority Shipping
Due to overwhelming demand, our initial shipments will go first to those who purchased in November. But don’t worry – our early bird customers will receive their devices quickly. We are doing everything possible to get RollnRest to you and your pets as fast as possible.
✔ Production Progress
PCB production is ongoing, packaging is ready, and final assembly is in full swing. Every step has been carefully executed to ensure your RollnRest meets the highest standards of quality and precision.
We are incredibly grateful for your patience, excitement, and trust throughout this journey. The moment you’ve been waiting for is almost here.
Get ready to experience RollnRest!
Date: February 3rd, 2026: RollnRest Production Update
We would like to share an update on our production timeline.
✔ PCB Production
PCB production is scheduled to begin on Friday, February 6th, in cooperation with our manufacturing partner. This is a key step toward final manufacturing and delivery.
✔ Delivery Schedule
Based on the current plan, deliveries are expected to begin on February 24th.
We will continue to keep you informed as production moves forward.
Thank you for your patience and continued trust.
Date: January 15th, 2026: RollnRest Production & Certification Update – Where We Are Now
We want to share a clear update on the current status of our production, certification, and delivery process.
✔ PCB Production in Progress
We have started pre-production of the lower PCB, an important step before moving into full-scale manufacturing. This phase allows us to validate quality and production parameters. Once completed, PCB production will transition to full production in cooperation with our manufacturing partner L-Tek Ltd.
✔ Packaging Preparation
At the same time, we are preparing all packaging components, ensuring everything is ready as soon as the control unit production is completed. This parallel approach helps prevent delays later in the process.
✔ FDA Registration & Compliance
All FDA registrations and required documents are up to date. This allows us to confirm that:
- The product is manufactured in an FDA-registered facility
- Manufacturing complies with FDA requirements
- Production meets FDA safety and quality system standards
- The product is listed with the FDA
✔ Medical Standards & Scientific Evidence
Our devices are produced according to medical-grade manufacturing standards. While we do not make medical claims, the effectiveness of the technology is supported by scientific studies.
✔ What’s Next
We are completing PCB pre-production and aligning packaging and manufacturing timelines. We will continue to share updates as we move closer to full production and delivery.
Thank you for your patience and trust.
Date: January 9th, 2026: PetsPEMF Development Update – EMC Testing Successfully Completed
We are pleased to share an important milestone in the PetsPEMF RollnRest development journey.
First of all, thank you once again for your continued patience, trust, and support. We are happy to report that we have now successfully completed the final certification phase.
✔ Final EMC Certification – PASSED
The final EMC (Electromagnetic Compatibility) test has been successfully completed and officially passed.
With this result, the EMC certification process is now fully concluded.
This marks a critical step forward, confirming that PetsPemf RollnRest meets all required regulatory and technical standards for production.
✔ Production Readiness Confirmed
With certification finalized, we are now moving forward without delay:
-
The second PCB is approved and ready for production
-
We are proceeding with full production preparation
✔ Production & Shipping Timeline
-
Production start: expected by January 20-25th, 2026
-
Production & assembly: approximately 1 week
-
Packaging & logistics: approximately 1 additional week
-
First units shipping: early February 2026
We are now entering the final stage before PetsPEMF RollnRest reaches you. Thank you for being part of this journey and for your trust throughout the development and certification process
We will share another update as production begins.
Date: January 6th, 2026; PetsPEMF Development Update
First of all, we would like to thank all our PetsPEMF customers for your patience, trust, and understanding throughout the Christmas and New Year holidays. We truly appreciate your continued support.
✔ Quality & Regulatory Update
PetsPEMF devices are developed and manufactured using the same medical-grade quality standards as our human devices, while using programs specifically designed for animals.
For U.S. market preparation, we have completed the GUDID database listing, which is required for device identification and import, even though regulatory pathways differ for veterinary products.
✔ Certification & EMC Testing Update
Following the holiday period, EMC testing has resumed.
The final EMC test is scheduled for January 9th, 2026.
✔ Next Steps
-
Final EMC test: January 9th, 2026
-
Review and confirmation of results
-
If everything is confirmed, we will proceed with production, final assembly, and packaging
Thank you once again for your patience and support over the holiday period. We look forward to sharing the next update very soon.
Date: Dec 29, 2025; RollnRest Development Update
First of all, we would like to thank all our customers for your patience, trust, and support over the Christmas and New Year period. We truly appreciate it.
✔ Certification & EMC Testing Update
Our certification body (SIQ) is currently on holiday for Christmas and New Year. As a result, EMC testing has been temporarily paused and is scheduled to resume on January 5th.
The next certification step is therefore expected from this date onward.
✔ Regulatory & U.S. Import Preparation
Although PetsPEMF devices are intended for animal (veterinary) use, we apply the same quality and safety standardsas for our human devices.
For U.S. market entry, we are now registering in the GUDID database, which is required for device identification and import into the United States, even when regulatory pathways differ for veterinary products.
In parallel, we are preparing all remaining documentation, labeling, and logistics needed for smooth U.S. import and distribution.
✔ Next Steps
- EMC testing resumes: January 5th, 2026
- Completion of EMC validation
- Final certification steps
- Transition to final production and packaging
We will continue to keep you informed as the next milestones are reached.
Date: December 19th, 2025; PetsPEMF Development Update — Quality & Production Standards
PetsPEMF devices are developed using the same medical-grade hardware quality and production standards as our human PEMF products.
What’s different are the programs — they are specifically designed for animals and adapted to their physiology, while the device quality, safety margins, and manufacturing process remain the same.
- Medical-grade hardware quality
- Animal-specific PEMF programs
- Same production partners & safety principles
- FDA registration — applies to human devices; veterinary devices follow a different regulatory pathway
By applying human-device engineering standards to PetsPEMF, we ensure the highest level of safety, reliability, and care for animals.
Thank you for your trust and continued support ❤️
Marko

Date: December 15th, 2025; NeoSurround Development Update — EMC Retesting in Progress
✔ EMC Testing Update
The EMC test conducted on December 8th, 2025 identified minor EMC-related deviations. As a result, a small EMC-specific technical modification is required, followed by a repeat EMC test.
The updated EMC solution has not yet been accepted, as final approval depends on the successful completion of the repeat EMC test. This retest represents the final outstanding step in the certification process.
Date: December 8th, 2025: RollnRest Development Update — Final EMC Testing & Preparation for Assembly
We are excited to share the latest progress on the RollnRest development and certification process. Our team has been working intensively over the past weeks to ensure the product meets the highest safety standards before entering full production.
✔ All RollnRest Beds Are Fully Prepared
The textile and foam components for every RollnRest bed have already been completed.
The beds are now waiting for final assembly with the electronic components.
✔ Coils Are Already In-House
All PEMF coil panels — the core therapeutic component of RollnRest — have been manufactured, delivered, and successfully passed internal quality testing.
They are ready for installation as soon as certification is finalized.
✔ Final EMC Test for the Control Unit
The control unit is currently undergoing the final EMC (Electromagnetic Compatibility) test as part of the IEC 60601 medical-grade safety certification.
This is the last step required before we can begin assembling the complete Roll&Rest system.
✔ Production Preparation Already Completed
To minimize delays, all other production steps have been prepared in advance:
-
Bed components → finished
-
Coils → in-house, tested
-
Packaging → ready
-
Assembly workflow → prepared
-
Documentation → finalized
Once EMC approval is received, we will begin assembly immediately.
✔ Estimated Delivery Window
We remain within the updated delivery timeframe of:
December 23rd – January 11
✔ What Comes Next
As soon as EMC confirmation arrives, RollnRest units will move straight into production and packaging.
We will continue to update this blog as we reach each milestone.
Thank you for your patience, trust, and enthusiasm for RollnRest. We are in the final stage of the certification journey and very close to delivering the product to you.
RollnRest Development Update — Final EMC Testing & Preparation for Assembly
Date: December 3rd, 2025
We are excited to share the latest progress on the RollnRest development and certification process. Our team has been working intensively over the past weeks to ensure the product meets the highest safety standards before entering full production.
✔ All Roll&Rest Beds Are Fully Prepared
The textile and foam components for every RollnRest bed have already been completed.
The beds are now waiting for final assembly with the electronic components.
✔ Coils Are Already In-House
All PEMF coil panels — the core therapeutic component of RollnRest — have been manufactured, delivered, and successfully passed internal quality testing.
They are ready for installation as soon as certification is finalized.
✔ Final EMC Test for the Control Unit
The control unit is currently undergoing the final EMC (Electromagnetic Compatibility) test as part of the IEC 60601 medical-grade safety certification.
This is the last step required before we can begin assembling the complete Roll&Rest system.
✔ Production Preparation Already Completed
To minimize delays, all other production steps have been prepared in advance:
-
Bed components → finished
-
Coils → in-house, tested
-
Packaging → ready
-
Assembly workflow → prepared
-
Documentation → finalized
Once EMC approval is received, we will begin assembly immediately.
✔ Estimated Delivery Window
We remain within the updated delivery timeframe of:
December 23rd – January 5th
✔ What Comes Next
As soon as EMC confirmation arrives, RollnRest units will move straight into production and packaging.
We will continue to update this blog as we reach each milestone.
Thank you for your patience, trust, and enthusiasm for RollnRest. We are in the final stage of the certification journey and very close to delivering the product to you.
December 3, 2025 — Redesign Completed & FDA Registration in Progress
Over the past weeks, our engineering and compliance teams have been working closely with the certification authorities to review every technical detail of our devices. During the IEC 60601 medical-grade certification process, the certification body identified a few areas where improvements were required to ensure even higher levels of safety, stability, and electromagnetic performance.
We are pleased to confirm that:
The redesign has been completed and submitted
A fully updated PCB and internal system redesign has now been delivered to the certification company.
This redesign addresses all issues identified by the certification authority, including:
-
electromagnetic susceptibility improvements
-
optimized safety margins
-
additional shielding
-
improved layout spacing
-
enhanced long-term reliability
These upgrades do not change the functionality of the product — they simply make the device even safer and more robust under medical-grade testing conditions.
FDA Device Registration Underway
We are also completing the necessary steps to register our devices with the U.S. FDA (Food and Drug Administration).
This includes:
-
device listing
-
documentation of manufacturing processes
-
safety and performance records
-
labeling and compliance alignment
This is an important milestone for both NeoSurround and RollnRest, allowing us to ensure full regulatory conformity in the U.S. market.
Shipping Window Remains
The updated estimated shipping window is:
December 23rd – January 5th
We will continue to post updates frequently as we progress toward full certification clearance and production start.
Thank you for your patience, trust, and support throughout this final stage of the certification journey.
November 20, 2025 — Final EMC Review & Control Board Revision
During the certification process for PetsPEMF RollnRest, SIQ requested a small revision to one section of our control PCB.
Their engineers identified a potential susceptibility area related to electromagnetic interference under extreme lab-test conditions.
While the device performs safely and normally in all everyday environments, SIQ recommended:
• improved spacing between two sensitive control components
• a cleaner grounding path
• and adding localized shielding
Our electronics team immediately implemented the redesign and prepared the updated board files for SIQ’s re-verification.
This adjustment adds approximately 24 days to our timeline — but ensures the device meets the same high safety and EMC standards as all our PEMF devices.
November 14, 2025 — Fabric & Foam Durability Tests Completed
Today we finished long-term mechanical testing of the Roll’n’Rest textile layers and foam structure.
Because this product is designed specifically for pets, the materials must withstand:
• scratching
• repeated pressure
• moisture exposure
• long-duration heat and PEMF cycles
• cleaning cycles
The neoprene/mesh combination passed all stress tests, and the internal foam retained its shape after over 35,000 compression cycles, simulating more than 2 years of daily use.
We also finalized the latest app integration. The RollnRest app experience will be nearly identical to the PetsPEMF app but optimized for automatic cycles and overnight operation.
November 4, 2025 — Pre-Production Assembly Planning
With the molds for textile cutting and the first batch of coils completed, today marks the start of our full pre-production planning phase.
We are aligning:
• coil assembly workflows
• fabric preparation
• electronics encapsulation
• quality-control points
• packaging and shipping procedures
In parallel, our team is filming short setup videos to help pet owners use RollnRest properly from day one.
October 24, 2025 — Certification Kick-Off (Pet Safety & EMC)
Earlier this month, we officially started the certification process.
Although RollnRest is a wellness device for animals, we follow the same safety and electromagnetic compatibility procedures as required for human-oriented PEMF devices.
This includes:
• electrical safety testing
• EMC field stability
• material safety and biocompatibility review
• long-duration operational temperature testing
We are preparing documentation for medical-grade IEC/EN standards used in PEMF equipment, ensuring Roll’n’Rest is safe for continuous contact with pets — including older dogs, sensitive animals, or those recovering from mobility issues.
September 14, 2025 — Coil Layout & Comfort Layer Validation
Around this time, we were finalizing one of the most important technical steps:
balancing coil performance with pet comfort.
Dogs have highly sensitive pressure points, so our engineering team worked intensively on:
• spacing coils evenly inside the mat
• ensuring no sharp edges or hard elements
• designing a smooth magnetic-field gradient across the entire surface
Early prototypes helped us validate that even large-breed dogs experience fully uniform PEMF intensity, even when lying curled, stretched, or partially off-center.
This stage directly shaped the final RollnRest architecture.
August 14, 2025 — Copper Coil Production Ordered
On this date, we placed the order for the custom coil sets powering the RollnRest system.
These coils were specifically designed for:
• deep-tissue PEMF penetration
• wide coverage for whole-body support
• low-heat, long-duration operation
• comfort-optimized dimensions to maintain softness
The supplier is now finishing the final production batch, which will be used in both pre-production and the first retail units.
You can pre-order your RollnRest soon.







Learn more about PetsPEMF devices: petspemf.com